Engaging value propositions in interactive customer communication tools
We help Pharmaceutical and Medtech market access teams develop engaging value propositions to communicate the clinical and financial value of their products using best-in-class digital tools.
We are leaders in:
- Payer value proposition development and presentation
- Simple yet robust budget impact models
- Real-world data visualisation dashboards (Connected Insights)
- Flashcards, leave-pieces, case studies and brochures
- Expert workshops and interviews for material scoping and validation
- Roll-out support and KAM training
- Target customer validation
- Local formulary / guideline / data review
- Advanced budgetary notifications (ABNs) and formulary packs
Locally relevant value communication for market access
In order to achieve local market access and optimise uptake, it is essential to communicate the clinical and financial value of your product in a way that is tailored and relevant to your customers.
Effective value communication materials enable Pharma and Medtech field teams to:
- Engage with payers and other healthcare decision-makers
- Clearly outline the evidence supporting their product
- Demonstrate the value the product delivers for patients and healthcare systems
To achieve this, we develop materials that are:
- Targeted to reflect local healthcare systems
- Rigorously evidenced-based
- Engaging and accessible
- Validated with customers
We validate key principles, messaging and designs directly with our network of payers, clinicians and other healthcare stakeholders. In the UK, we have close relationships with over 90 contracted Associates working in the NHS, who work directly with us on our value communication materials to ensure our messages will resonate with decision-makers.
Service and treatment pathway optimisation
Successful local access for a pharmaceutical product or medical device can be impacted by the state of the service in which patients are managed. Capacity, workforce and financial challenges can all limit the ability of a service to identify, diagnose, treat and monitor patients.
Highlighting and quantifying these challenges, and making recommendations for addressing them, can form a valuable part of a local access campaign. We can support you by developing tools and materials such as white papers, case studies and capacity calculators, for use with local service providers and policy makers.
Developing value communication tools
We work with you to develop and refine your value proposition for local markets and identify the most impactful communication materials for your product and audience.
We typically apply this simple project framework:

Step 1: Scoping & research
Our market access specialists begin by reviewing your existing materials and data, along with relevant guidelines, clinical standards and policy documents.
We then facilitate a workshop with your team to understand your commercial needs and your product’s value proposition, and to begin mapping out the key messages, content and structure of your tool.
Step 2: Expert input
We strengthen and supplement this research and our initial ideas for the project with our broad network of payers, clinicians and other healthcare stakeholders. In the UK, this includes over 90 contracted Associates working in the NHS, who work with us directly on projects.
This process may involve pathway mapping to understand how your treatment can reach patients and where there may be opportunities to support local health services to implement change and improvements in care for patients.
Step 3: Storyboard and content
We then present our recommendations for your communication tool. This will include a storyboard for initial review and approval, before progressing to development of full content, including structure, key messages, data and references.
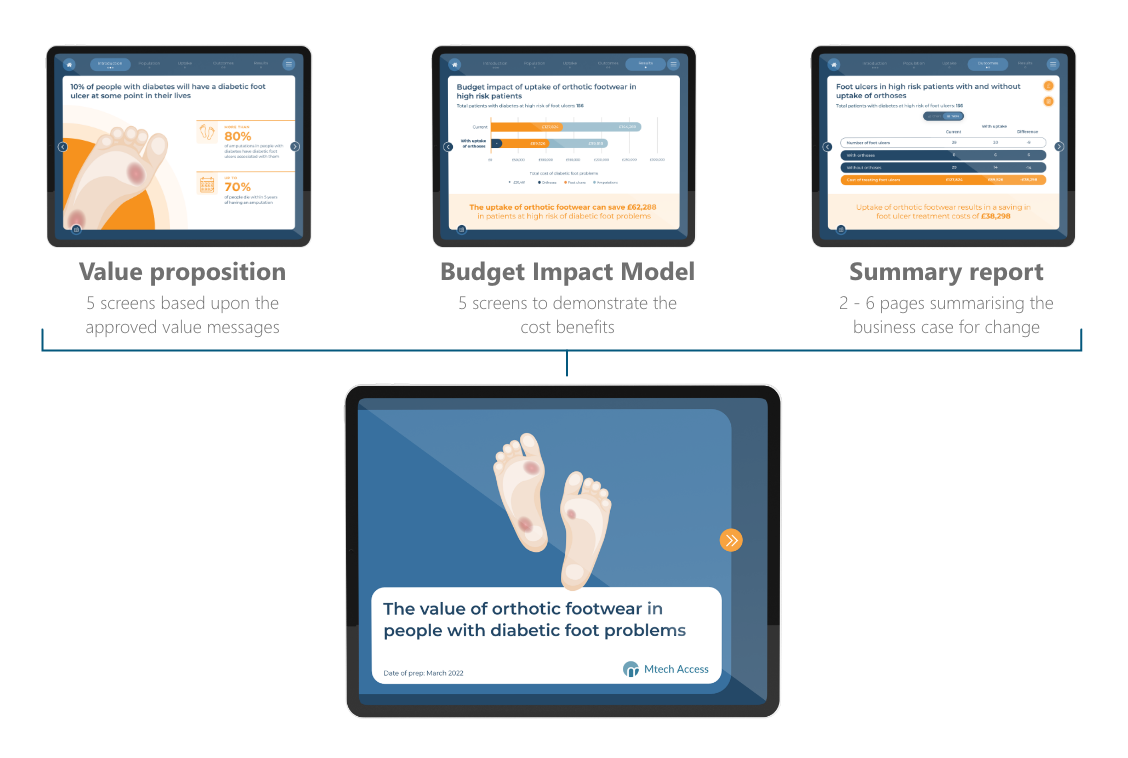
A typical payer toolkit will comprise a value proposition introduction, a budget impact model demonstrating the financial and/or clinical benefits of your intervention, and a dynamic summary report summarising the business case for change. We can develop your messaging and health economic model from scratch, or we can adapt messaging and models developed at a global level or for Health Technology Assessment (HTA) submission, for use locally.
Our value communication experts and health economists ensure the content is robust, evidence-based, engaging and easy for field teams and healthcare professionals to navigate.
Step 4: Digital development
After approval of tool content, we begin digital development of your communication tool.
Our in-house design and digital teams create best-in-class digital tools that can be housed on the iPad, presented remotely via the web or integrated with CRM systems such as Veeva, and incorporate graphics, interactivity and layering for a transparent, engaging presentation. Our digital tools adhere to your branding guidelines and are compliant with relevant regulations and codes, such as the ABPI Code of Practice.
Step 5: KAM training & roll-out
Our aim is to ensure your communication tool works for your field-teams, enables Key Account Managers to engage with healthcare decision-makers, and ultimately helps your product reach patients. A key part of this is a structured KAM training and roll-out programme.
We can provide detailed user guides and training sessions to set up KAMs for success. We can also deliver in-person or remote training sessions, supported by a relevant NHS Associate providing customer insights and guidance. Training can include:
- How to navigate the tool to present the story, input data, and display key information and references
- Exercises/scenarios to practice using the tool with different audiences
- Role play
- Objection handling and best-practice workshops
Watch our video on Digital Customer Communication Tools for Market Access
Digital value communication tools
Discover how we develop digital value communication tools to meet the needs of internal stakeholders and healthcare customers.
Featured Case Studies
-
View Case Study
Developing a digital toolkit to demonstrate the value of a pharmaceutical technology to secondary care providers
-
View Case Study
Delivering a White Paper to highlight national variation in care, and propose solutions for improving care across the NHS
-
View Case Study
Using real-world data to build a case for change
-
View Case Study
Giving healthcare customers a true picture of budget impact
-
View Case Study
Optimising visualisation and communication in an app-based Cost-Consequence Model
Why choose Mtech Access for your next customer communication project?
- Our team have 180+ years’ collective experience creating engaging local value propositions, budget impact models and customer communication resources that resonate with healthcare decision-makers*
- At Mtech Access, we have worked on 275+ projects with 40+ clients†
- Our in-house developers and designers collaborate directly with our value communication experts, health economists and medical writers to create your materials
- We validate the principles, messages and designs of our customer communication materials with a broad network of payers, clinicians and other healthcare stakeholders across global markets
- In the UK, we have close relationships with over 90 contracted Associates occupying roles in the NHS, who work directly with us on projects to help us ensure our communication materials will resonate with NHS decision-makers
- We combine evidence and creativity, to deliver resources that work for your field-based teams
- We are a full-service Veeva partner, able to leverage Veeva’s full functionality to deliver the best possible experience for your teams and customers
- Our team have 160+ years’ collective experience creating engaging local value propositions, budget impact models and customer communication resources that resonate with healthcare decision-makers*
- At Mtech Access, we have worked on 160+ projects with 30+ clients†
- Our in-house developersand designers collaborate directly with our value communication experts, health economists and medical writers to create your materials
- We validate the principles, messages, and designs of our customer communication materials with a broad network of payers, clinicians and other healthcare stakeholders across global markets
- In the UK, we have close relationships with over 80 contracted associates working in the NHS, who work directly with us on projects to help us ensure our messages will resonate with NHS decision-makers
- We combine evidence and creativity, to deliver resources that work for your account managers
- We are a full-service Veeva partner, able to leverage Veeva’s full functionality to deliver the best possible experience for your teams and customers
*Includes senior team members’ experience prior to joining Mtech Access.
†Includes projects involving the development of a value proposition, budget impact model, customer messaging or a customer communication tool for an external Pharmaceutical, Medtech, Biotech or Diagnostics client.
Articles related to our Customer Communication materials
Digital options to enhance your local market access materials
How can a digital approach enhance local customer communication, particularly when tools are adapted from existing global materials?
6 questions to ask when adapting global market access materials for local markets
How can you ensure that the market access materials, value proposition and evidence developed by global teams, will resonate for your local audience? In this article, our experts pool their knowledge to share their top tips for successfully taking global materials local…
6 top tips for getting a new diagnostic into the NHS
Understanding the diagnostic landscape in the UK: challenges facing comercialisation teams and top tips to achieve market access…